
The crystalline structure of cellulose has been studied since its discovery in the 19th century. Thorough reviews of cellulose crystalline allomorphs can be found elsewhere. Celluloses IV I and IV II can be obtained by heating celluloses III I and III II, respectively. Celluloses III I and III II can be formed from celluloses I and II, respectively, by treatment with liquid ammonia, and the reaction is reversible. Cellulose II can be prepared by two distinct routes: mercerization (alkali treatment) and regeneration (solubilization and subsequent recrystallization). Cellulose I is the most abundant form found in nature. Four different crystalline allomorphs have been identified by their characteristic X-ray diffraction (XRD) patterns and solid-state 13C nuclear magnetic resonance (NMR) spectra: celluloses I, II, III and IV. Hydroxyl groups present in cellulose macromolecules are involved in a number of intra- and intermolecular hydrogen bonds, which result in various ordered crystalline arrangements. The repeating unit of cellulose is cellobiose. In addition, the prediction of cellulase performance based on low levels of cellulose conversion may not include sufficient digestion of the crystalline component to be meaningful.Ĭellulose is a high molecular weight linear polymer composed of D-glucopyranose units linked by β-1,4-glycosidic bonds. Given the methodological dependency of cellulose CI values and the complex nature of cellulase interactions with amorphous and crystalline celluloses, we caution against trying to correlate relatively small changes in CI with changes in cellulose digestibility. Cellulose accessibility should be affected by crystallinity, but is also likely to be affected by several other parameters, such as lignin/hemicellulose contents and distribution, porosity, and particle size. Although celluloses having a high amorphous content are usually more easily digested by enzymes, it is unclear, based on studies published in the literature, whether CI actually provides a clear indication of the digestibility of a cellulose sample.
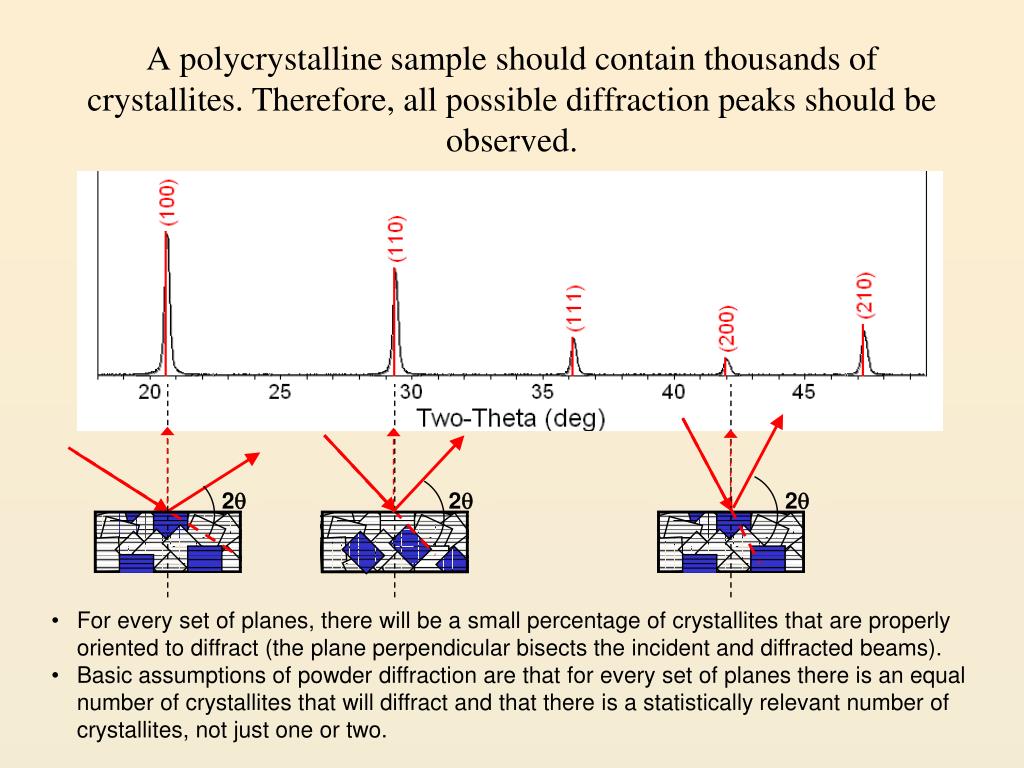
We believe that the alternative X-ray diffraction (XRD) and NMR methods presented here, which consider the contributions from amorphous and crystalline cellulose to the entire XRD and NMR spectra, provide a more accurate measure of the crystallinity of cellulose. Data in the literature for the cellulose preparation used (Avicel PH-101) support this observation. We found that the simplest method, which is also the most widely used, and which involves measurement of just two heights in the X-ray diffractogram, produced significantly higher crystallinity values than did the other methods. In this study, four different techniques incorporating X-ray diffraction and solid-state 13C nuclear magnetic resonance (NMR) were compared using eight different cellulose preparations. Although measurements of crystallinity index (CI) have a long history, it has been found that CI varies significantly depending on the choice of measurement method.


 0 kommentar(er)
0 kommentar(er)
